How does OncoAct work?
From biopsy to report
What happens to your tumor tissue and blood during a comprehensive DNA test? What exactly does all the complex equipment do? How do you know which treatments might be an option for you? Watch the video now!
Whole genome sequencing

OncoAct uses whole genome sequencing which makes it possible to read the complete DNA of the tumor tissue and blood.
Human DNA, also called the genome, consists of 2×3 billion “letters” (one copy from your mother and one from your father) and is present in every somatic cell. This DNA contains all the information necessary for a cell, tissue, and organism to function and for cells to divide.
Coincidental and environmental factors can damage the DNA. This can change the properties of a cell and cause cancer. For a good understanding and optimal treatment of a tumor, it is therefore important to have a high resolution “photo” of the damaged DNA from the tumor.
DNA damage occurs in all kinds of ways. It may be that some DNA letters have changed, but also that small or large pieces of DNA have disappeared or doubled. DNA can also break down and be repaired in the wrong way by the body, after which it ends up in another place in the genome.
Whole genome sequencing can be used to track all types of changes. To ensure that the changes measured are mutations that only occur in the tumor, the DNA of the tumor is always compared with the “normal” DNA of the patient. For this we use DNA from the blood.
Patient report
After an extensive bioinformatic data analysis, we examine which existing and experimental treatments match the characteristics (biomarkers) of the tumor, both for registered and off-label indications. We use global knowledge gathered in specialized databases and overviews of ongoing research in the Netherlands to do this.
All relevant molecular data and treatment options are summarised in the OncoAct patient report. This gives the treating physician as complete an overview as possible of the cancer-related DNA mutations in the patient.
The OncoAct WGS report contains the following detailed information:
- a general summary of relevant clinical evidence
- available medication that may work for the DNA mutations that were found
- extensive molecular details to support a treatment choice
The OncoAct WGS report also provides insight into:
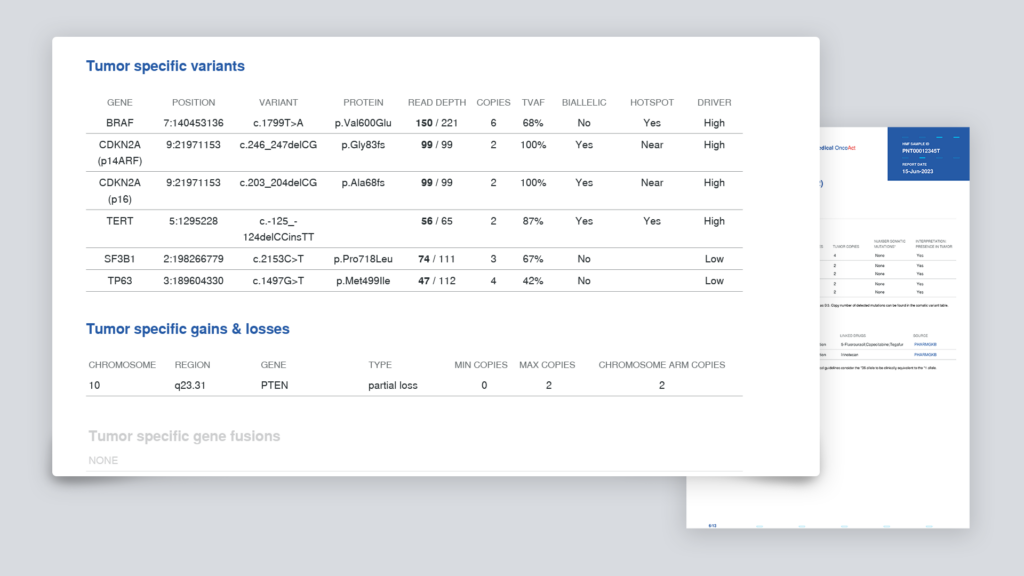
- tumor-specific mutations, amplifications, deletions
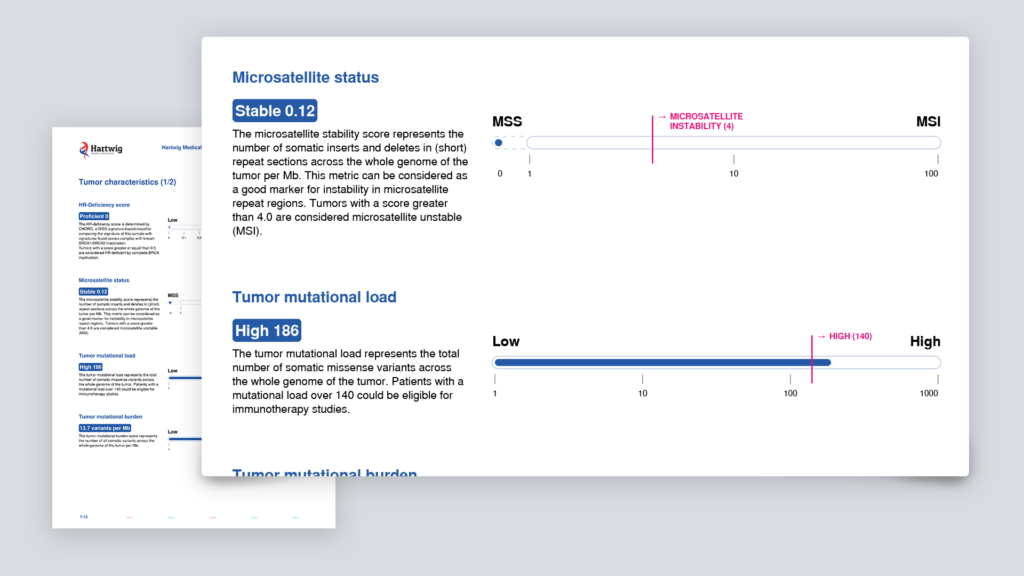
- mutational load/mutational burden
- microsatellite instability
- presence of fusion genes as a result of translocations
- the opportunity to participate in clinical studies in the Netherlands
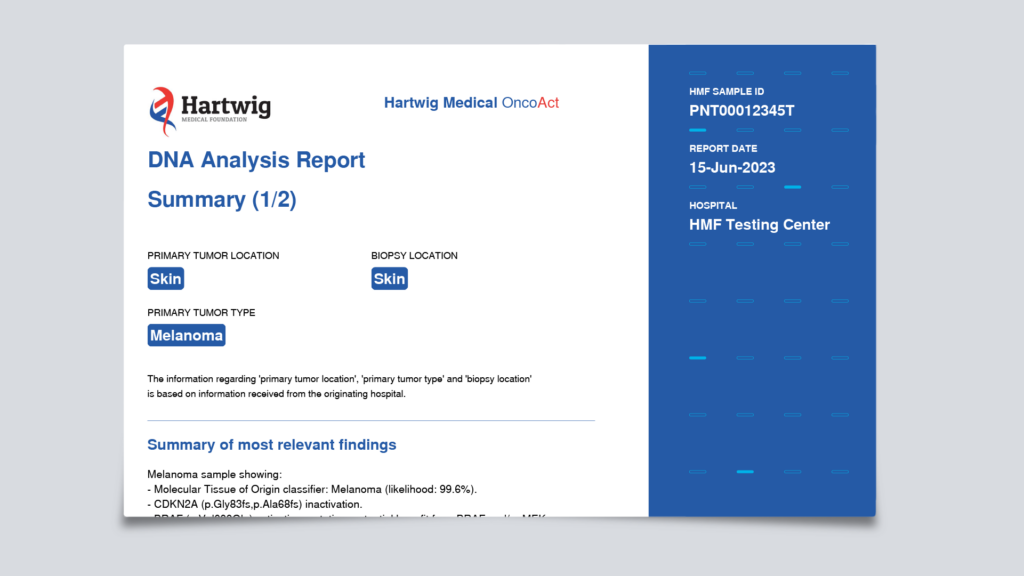
The summary contains an overview of genes with potential actionable mutations and various general tumor characteristics, such as microsatellite instability (MSI) and tumor mutational load (TML). These characteristics are important for immunotherapy options.
Click here for an example of the OncoAct WGS report.
What is included in the OncoAct WGS report?
The results of all existing DNA tests in one report

What is included in the OncoAct WGS report?
A summary of the outcomes that are relevant to a possible treatment
What is included in the OncoAct WGS report?
An extensive representation of all changes in the hereditary material (mutations)
What is included in the OncoAct WGS report?
Results that have always been tested against the most recent scientific insights
How does it help you?
The report supports the choice of treatment
- standard treatments
- available experimental studies
- off-label medication

Advantages of a DNA Test Based on Whole genome sequencing
All possible biomarkers
All possible DNA-based biomarkers are determined in one DNA test
One report
All results and outcomes are summarised in one report
One overview
Relevant results with possible treatment options are summarised in one overview
Patients Were Analysed
In recent years, we have analysed more than 5,800 patients with metastatic cancer using whole genome sequencing.
The number of results that may be relevant for treatment varies greatly between cancer types.
Existing Medication
On average, we found indicators for treatment with existing medication for 31% of patients.
Off-Label medication
In 13% of these patients, this is medication that is actually registered for another tumour type (off-label)*.
* published in Nature, November 2019
Experimental Medication
For approximately 31% of patients we also found indications for using experimental medication. Access to these medications is possible through clinical trials.
Off-Label Treatment
A recent study shows that 34% of patients benefit from off-label treatment in clinical trials.*
* published in Nature, September 2019
Patient report
Treatment Advice Following Complete DNA Analysis of Patients over the Past 4 Years
Duration
It is important for the patient and the treating physician to know the results of OncoAct quickly.
In general, we send a patient report to the treating physician within 10 business days after receiving the tumor tissue and blood, if the tumor tissue and blood are of sufficient quality.
The DNA Testing Process
The tumor tissue and blood are immediately scheduled for processing after receipt and registration. Processing the tumor tissue and blood consists of a number of labour-intensive steps, each of which takes one day or more: DNA isolation, sample preparation, DNA sequencing, and bioinformatic data analysis. We conduct quality checks after each step. If the tumor tissue contains sufficient tumor cells and the DNA is of sufficient quality, we perform the test and can report the results. If this is not the case, we do not produce an OncoAct WGS report.
Validating the DNA Test
OncoAct is performed according to standardised procedures in the ISO17025 accredited laboratory of Hartwig Medical Foundation in Amsterdam. The methods used and test results have been extensively validated. You can request these by e-mail.